
In today’s article, we’ll take a look at the refractory alloy sintering process. Refractory alloy sintering can be divided into six stages.
With the rising temperature, the molding agent gradually decomposes or vaporizes with the sintered body left. At the same time, the molding agent more or less adds carbon to the sintered body. The rising amount of carbon changes with the types and quantities of molding agents as well as the different sintering methods. The surface oxide of the powder can be reduced.
If the molding agent is removed and the carbon-oxygen reaction is not strong, hydrogen can be used to reduce the oxidation of cobalt and tungsten at sintering temperature. The contact stress among powder particles gradually disappears. Bonding metal powder began to produce recovery and recrystallization. Surface diffusion began to occur and briquette strength improves.

Refractory Metal
At the former temperature before the liquid phase, the last period’s reaction continues. Meanwhile, solid-phase reaction and diffusion get intensified. The plastic flow becomes more violent and the sintered body significantly shrinks.
When the sintered body enters into the liquid phase, the shrinkage is almost completed followed by a crystal transition to form the basic structure and structure of the alloy.
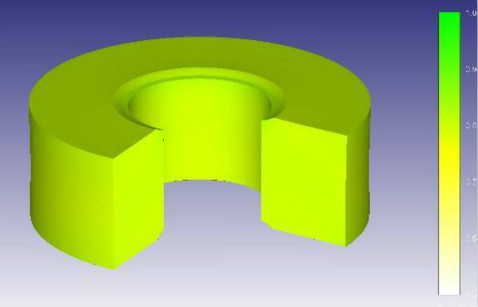
Liquid Phase Sintering
At this stage, the organization and phase composition of the alloy can change with different cooling conditions. Therefore, this feature can be used to improve the alloy’s physical and mechanical properties with heat processing.
Infiltration is an important factor in the liquid phase sintering process. It refers to the infiltration capacity of liquid to the solid. If a drop of liquid can be completely dispersed on the surface of the solid when dropping on the solid, then the liquid has infiltration capacity and vice versa.
If the liquid can only wet parts of the solid, then it has the partial capacity for liquid infiltration. If the liquid metal can completely wet the surface of solid particles during the liquid phase sintering, the sintered body will have small pores. If the wetting capacity is not ideal, there will be many sintered body defects.
During the sintering process, cemented refractory alloy compacts usually have significant shrinkage. The shrinkage of the sintered body can be divided into three basic stages. During the first stage with the temperature under 1150℃, the sintered body has a shrinkage phenomenon.
However, the shrinkage in this period only takes a few percentages. The sintered body has great shrinkage at the second stage with a temperature of over 1150℃. The degree of shrinkage can reach 80% of the total. The sintered body becomes completely dense after a small percentage of shrinkage in the liquid phase.
There are many factors affecting shrinkage in the refractory alloy sintering process, the most common ones are listed below.
The shrinkage will be in accordance with the three shrinkage stages mentioned above if the heating rate is normal, like several degrees’ rise per minute. However, if the heating speed is too fast, the shrinkage velocity will reach a maximum at a higher temperature than in the second stage. It has been found that a high heating rate will cause e a large number of coarse pores and bubbles in the alloy because the gas discharge channels are closed in the liquid phase. Therefore, the excessive heating speed is not good for producing completely compact sintered bodies.
When briquettes are sintered in an inert atmosphere, the shrinkage rate will increase with the reduction of the briquette density. The relative shrinkage and relative shrinkage speed of the briquette with different densities are the same. The final density of the alloy is irrelevant to the original pores in the compact. However, when sintered in an active atmosphere, it is difficult to produce a high-density sintered body with large porosity. Therefore, the density of the compacts needs to be improved as strongly as possible in actual work.
The smaller the sizes of refractory alloy particles are, the smaller the individual pores in the sintered body are. The capillary pressure of the liquid is inversely proportional to the radius of the pores. The distance between the two refractory alloy particles is shortened with the decrease of particles’ amounts. Therefore, small particles are likely to get close during sintering.
Besides, powders with larger surfaces have faster solid-phase diffusion rates, rearrangement rates, and dissolution rates. Therefore, the grinding mixture and original crystal grains have different shrinkage qualities from the general mixtures. The temperature at which the shrinkage begins has a significant reduction while the shrinkage speed is greatly improved before the liquid phase.
Shrinkage
There is no doubt that the cobalt content has an effect on the shrinkage after the liquid phase. The higher the cobalt content is, the higher the shrinkage rate is. Experiments show that the increase of cobalt quantity in the compact can hinder the shrinkage at the first stage. But it can greatly promote the second phase shrinkage because the contraction mechanism is plastic flow and the increase of cobalt content will promote plastic flow.
The carbon content of the sintered body affects the initial temperature of the liquid phase and the amount of the liquid phase. Therefore, the carbon content influences the shrinkage of the whole sintering process. In theory, the excess carbon content of the mixture not only promotes the third-stage shrinkage but also promotes the second-phase contraction.
Thank you for reading our article and we hope you’ve enjoyed it. If you want to know more about refractory metals, you can visit Advanced Refractory Metals for more information. We provide our customers with high-quality refractory metals at a very competitive price.
Copyright © 1994-2024 Advanced Refractory Metals owned by Oceania International LLC, All Rights Reserved.