Hafnium Oxide And Its Structure & Applications
Hafnium Oxide and Its Structure And Applications
Hafnium oxide is the inorganic compound of the formula HfO2. Also known as hafnia, this colorless solid is one of the most common and stable compounds of hafnium. It is an electrical isolator with a bandgap of 5.3 ~ 5.7 eV. Hafnium dioxide is an intermediate in some processes that give hafnium metal.
Structure And Applications Of Hafnium Oxide
Hafnium oxide is quite inert. It reacts with strong acids such as concentrated sulfuric acid and strong bases. It dissolves slowly in hydrofluoric acid to give fluorohafnate anions. At high temperatures, it reacts with chlorine in the presence of graphite or carbon tetrachloride to give hafnium tetrachloride.
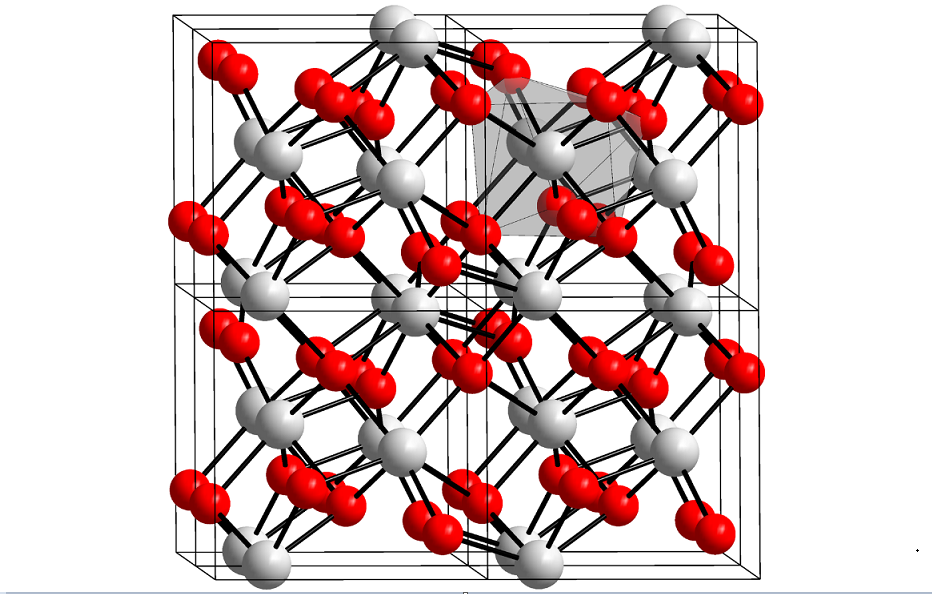
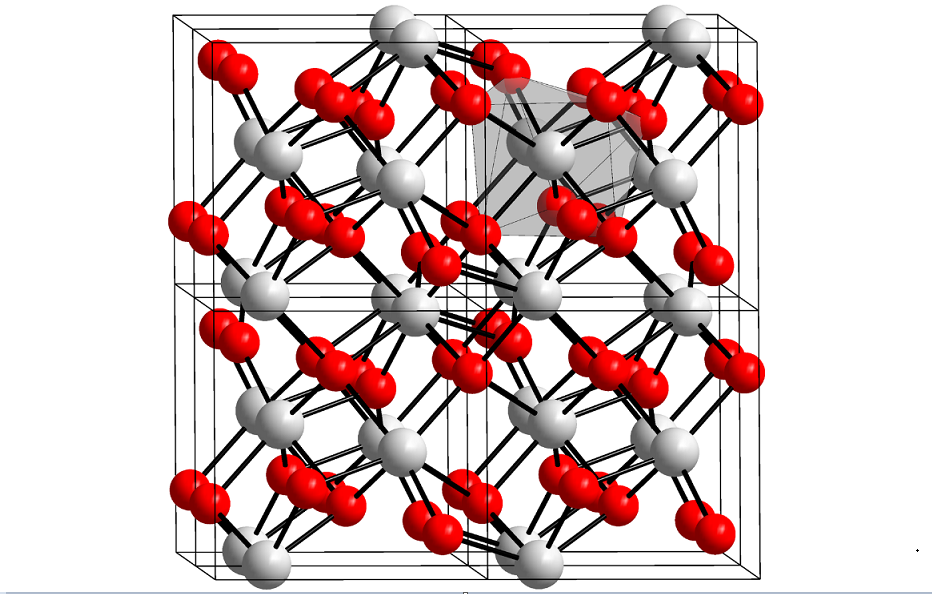
HfO2 adopts the same structure as zirconia (ZrO2). Unlike TiO2, which includes six-dimensional Ti in all phases, zirconia, and hafnia consist of seven-coordinate metal centers. A variety of crystalline phases were observed experimentally, including cubic (Fm-3m), tetragonal (P42 / nmc), monoclinic (P21 / c), and orthorhombic (Pbca and Pnma).
It is also known that hafnia can adopt two other orthorhombic metastable phases (space group Pca21 and Pmn21) over a wide range of pressures and temperatures, presumably the sources of ferroelectricity recently observed in hafnia thin films.
Thin films of hafnium oxides, used in modern semiconductor devices, are often deposited with an amorphous structure (usually by atomic layer deposition). The possible advantages of the amorphous structure led the researchers to combine hafnium oxide with silicon (forming hafnium silicates) or with aluminum, which proved to increase the crystallization temperature of the oxide. hafnium.
Hafnia adopts the same structure as zirconia (ZrO2). Unlike TiO2, which includes six-dimensional Ti in all phases, zirconia, and hafnia consist of seven-coordinate metal centers. A variety of crystalline phases were observed experimentally, including cubic (Fm-3m), tetragonal (P42 / nmc), monoclinic (P21 / c), and orthorhombic (Pbca and Pnma).
It is also known that hafnia can adopt two other orthorhombic metastable phases (space group Pca21 and Pmn21) over a wide range of pressures and temperatures, presumably the sources of ferroelectricity recently observed in hafnia thin films.
Thin films of hafnium oxides, used in modern semiconductor devices, are often deposited with an amorphous structure (usually by atomic layer deposition). The possible advantages of the amorphous structure led the researchers to combine hafnium oxide with silicon (forming hafnium silicates) or with aluminum, which proved to increase the crystallization temperature of the oxide. hafnium.
Hafnia is used in optical coatings and as a high-K dielectric in DRAM capacitors and in advanced metal oxide semiconductor devices. The hafnium oxides were introduced by Intel in 2007 to replace silicon oxide as a gate insulator in field-effect transistors.
The advantage for the transistors is their high dielectric constant: the dielectric constant of HfO2 is 4-6 times that of SiO2. The dielectric constant and other properties depend on the deposition method, the composition, and the microstructure of the material.

Hafnium Oxide
In recent years, hafnium oxide is attracting additional interest as a possible candidate for resistive switching memories and a CMOS-compatible ferroelectric memory.
Because of its very high melting point, hafnia is also used as a refractory material in the insulation of devices such as thermocouples, where it can operate at temperatures up to 2500 ° C.
Multilayer films of hafnium dioxide, silica, and other materials have been developed for use in the passive cooling of buildings. The films reflect sunlight and radiate heat at wavelengths that cross the Earth's atmosphere and can have temperatures several degrees cooler than surrounding materials under the same conditions.
Conclusion:
Thank you for reading our article and we hope it can help you to have a better understanding of hafnium oxide and its structure and applications. If you want to know more about hafnium and other refractory metals, you can visit Advanced Refractory Metals (ARM) for more information.
Headquartered in Lake Forest, California, USA, ARM is a leading manufacturer & supplier of refractory metals across the world, providing customers with high-quality refractory metal products such as tungsten, molybdenum, tantalum, rhenium, titanium, and zirconium at a very competitive price.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






