Nano Tungsten Carbide - The Platinum-like Catalyst

Nano Tungsten Carbide - The Platinum-like Catalyst
In nature, platinum group elements together with gold and silver are known as precious metal elements. The platinum family has platinum, iridium, rhodium, and palladium. The most important value of platinum is not that it is platinum jewelry, but that it is an important industrial catalyst, such as in the aerospace fuel and new energy fuel cells industries.

Tungsten Carbide
Platinum is useful but rare. According to the survey, 70% of the world's platinum is produced in South Africa and is expensive, which often limits the development and progress of high-tech industries. Therefore, it is especially important to find an element that can replace the platinum catalyst. In this article, let's take a look at the nano tungsten carbide - the platinum-like catalyst.
Tungsten carbide cemented carbide has special corrosion resistance, high hardness, excellent fracture toughness, and compressive strength, which are called “Modern Industrial Teeth”.
The development of Nano-composite technology and materials has injected new vitality into it, and its application fields have been extended from ordinary tools, molds, wear-resistant parts, and wear-resistant coatings to large-scale integrated printed circuit boards, micro-drill bits, point-array printers that can withstand hundreds of millions of hits, precision tooling, hard material cutting tools, high-strength wear-resistant parts, geological drill bits, and military weapons, etc.
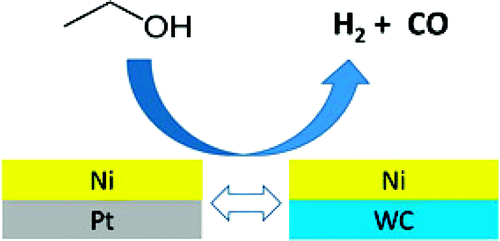
However, what is less well known is that Nano tungsten carbide also has catalytic properties similar to platinum.
A large number of research results show that the surface composition of Nano tungsten carbide has a great influence on its catalytic performance in the hydrolysis of hydrocarbon and its adsorption of hydrogen in the process of reaction.
The catalytic effect of tungsten carbide on the hydrolysis of hydrocarbon is based on a dual functional structure on the surface of tungsten carbide, namely the acid center formed by the presence of oxygen on the surface of tungsten carbide and the metal point formed by tungsten carbide.
In the process of catalyzing hydrocarbon reaction, metal points can strongly adsorb hydrogen and hydrocarbon molecules in the reactant to form their respective active groups on the surface of tungsten carbide, while acid WOX can promote the change of carbon chain structure and generate isomerized products, as well as prevent further hydrolysis of the isomerized product from the active center of tungsten carbide.
Nano tungsten carbide
It can be seen that the Nano tungsten carbide with its special platinum-like characteristics is expected to replace platinum and become a new generation of aerospace fuel and new energy cell catalyst. Through continuous improvement of the preparation methods and composite methods of tungsten carbide powder, high-end energy materials will become more and more popular in the future, and thousands of petrochemical materials in households will be replaced.
Summary
Thank you for reading our article and we hope it can be helpful to you. If you want to know more about tungsten and nano tungsten carbide, you can visit Stanford Advanced Materials for more information. They offer high-quality tungsten products to meet customers' R&D and production needs.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






