Noble Metal Compounds: Varieties and Applications
Introduction
Noble metals involve elements such as gold (Au), silver (Ag), platinum (Pt), and palladium (Pd). They have long caught human beings not only for their lustrous beauty but also for their exceptional chemical properties. Unlike most metals, noble metals resist corrosion and oxidation in moist air, so they have become invaluable across a broad spectrum of applications.
This article delves into the diverse types of noble metal compounds and their wide-ranging applications. Hope that you can learn about how they impact medical treatments, environmental solutions, technology, and renewable energy.
Understanding Noble Metal Compounds
Noble metal compounds are chemical compounds that include at least one noble metal atom in their structure. These compounds leverage the unique properties of noble metals, such as excellent conductivity, chemical stability, and catalytic activity. They come in various forms, including simple ions, complex ions, organometallic compounds, and nanoparticles. Each of them comes with distinct advantages for different applications.
Types of Noble Metal Compounds
Here's a closer look at the types of noble metal compounds and their distinctive characteristics:
1. Gold (Au) Compounds:
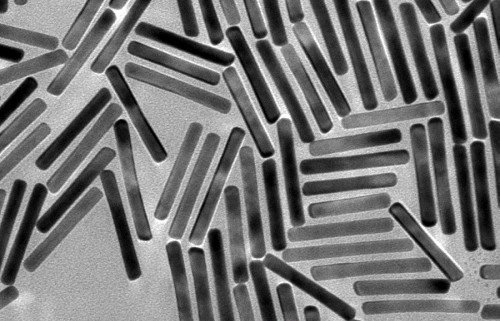
- Gold Nanoparticles (AuNPs) are utilized across electronics, catalysis, and biomedicine. These compounds stand out for their exceptional optical properties and expansive surface area.
- Gold Chlorides (AuCl3) find applications in organic synthesis as catalysts, besides their use in electroplating and as colorants in ceramics.
Gold Nanorods
2. Silver (Ag) Compounds:
- Silver Nitrate (AgNO3) serves as a precursor to various silver compounds, with notable applications in photography, mirror manufacturing, and as antiseptics.
- Silver Sulfadiazine (AgC10H9N4O2S) is widely applied in the treatment of burns due to its antimicrobial properties.
- Silver Nanoparticles come with their antibacterial effects and are incorporated into coatings, textiles, and medical devices.
3. Platinum (Pt) Compounds:
- Cisplatin (Pt(NH3)2Cl2) is employed as a chemotherapy drug to treat a range of cancers.
- Platinum Oxides, including PtO and PtO2, act as catalysts in several chemical reactions, notably in fuel cells and for reducing vehicle emissions.
- Platinum Black, a fine platinum powder, is useful in electrodes for its significant surface area.
4. Palladium (Pd) Compounds:
- Palladium(II) Oxide (PdO) serves as a catalyst and a starting material for synthesizing other palladium compounds.
- Palladium(II) Nitrate Hydrate frequently acts as a catalyst in organic synthesis and for creating palladium-based catalysts.
- Palladium(II) Acetate is essential in catalysis, particularly in cross-coupling reactions.
- Palladium(II) Chloride finds use in catalytic processes and as a precursor for other palladium compounds.
- Palladium(II) Sulfate is used in plating and as a catalyst in various chemical reactions, including those involving organic synthesis.

Palladium(II) Nitrate Hydrate
5. Rhodium (Rh) and Iridium (Ir) Compounds:
- Rhodium Complexes, like RhCl(PPh3)3, are employed in the hydroformylation of alkenes.
- You can find Iridium Oxides (IrO2) in electrochemical applications, including the water-splitting process for hydrogen production.
- Iridium Complexes find uses in organic light-emitting diodes (OLEDs) and as catalysts in water oxidation reactions.
6. Organometallic Compounds:
Organometallic compounds feature noble metals bonded to organic ligands, and they play a crucial role in catalysis, pharmaceutical synthesis, and material science. Their versatility in facilitating a wide range of chemical transformations makes them highly valuable.
Applications across Fields
The unique properties of noble metal compounds have led to their integration into numerous fields, so they significantly impact technology, healthcare, and environmental sustainability.
--Medical Innovations
Noble metal compounds have revolutionized the medical field. Gold and platinum compounds are pivotal in cancer therapy. They are the basis for chemotherapeutic agents that target and destroy cancer cells.
For instance, silver compounds come with their potent antimicrobial properties. They are incorporated into dressings and coatings to prevent infections in wounds and medical devices.
--Technological Advancements
In technology, the conductive properties of noble metal compounds find applications in electronics and computing. Gold and silver nanoparticles are useful in conductive inks for printed electronics, while palladium compounds play a critical role in the manufacturing of electronic components.
--Environmental Solutions
Noble metal compounds contribute significantly to environmental protection efforts.
- Platinum and palladium catalysts are at the heart of catalytic converters in vehicles. They reduce harmful emissions and combat air pollution.
- Additionally, noble metals are crucial in the development of green chemistry, catalyzing reactions that minimize waste and energy consumption.
--Renewable Energy
The push towards renewable energy sources has benefited from noble metal compounds. Platinum and palladium catalysts enhance the efficiency of fuel cells. These catalysts promise cleaner alternatives to fossil fuels. Gold and silver compounds are integral to the improvement of photovoltaic cells for solar energy. So, renewable sources become more viable and efficient.
Challenges and Future Directions
Despite their invaluable contributions, the use of noble metal compounds is not without challenges. High costs, limited availability, and the environmental impact of mining pose significant hurdles.
Future research is focused on developing sustainable synthesis methods, recycling noble metals from electronic waste, and finding cheaper, abundant alternatives that mimic the properties of noble metals.
Conclusion
Noble metal compounds stand at the intersection of tradition and innovation, offering a bridge from the past to a sustainable future. Their roles in healthcare, technology, environmental sustainability, and renewable energy highlight their indispensability and the continuous need for research and development in this area.
As we uncover more about these remarkable compounds, their potential to drive progress and solve some of the world's most pressing challenges becomes ever more apparent. They are marking a promising path for the future of science and technology.
Advanced Refractory Metals (ARM) specializes in manufacturing high-purity precious metal compounds, catering to catalytic, electronic, pharmaceutical, and research & development sectors. We customize our precious metal compounds to meet your specific needs. Visit ARM for more information.
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






