Comparing the Melting Points of Alloys with Their Constituent Metals

1. Introduction to Alloys
An alloy is a blend of 2 or more chemical elements, one of which is supposed to be metal. Different substances of controlled amount are added to a metal to form alloys with desirable properties. Examples of alloys like stainless steel, bronze, and brass are applied to various fields including construction, automobiles, medical, and aerospace industries.
Let’s have a further discussion about alloys and their properties, and this article will start by comparing the melting points of alloys with these of their constituent metals.

2. The Melting Point and Melting Range
Melting point is a significant feature of alloys and metals. It is the temperature at which a substance changes from a solid state to a liquid state. At this temperature, the solid state and liquid state coexist in equilibrium.
Unlike pure metals, most alloys have a melting range rather than a melting point, during which the substance experiences a slush transition between the solid state and the liquid state. The following is a further explanation of the alloy material melting process:
- Alloys begin to melt at the solidus temperature and the substance is partly liquid and partly solid.
- They finish melting at the liquidus temperature.
- There are special cases where some alloys change their state at one single temperature, which is called the eutectic temperature.
- The wider the melting range is, the more impure the substance is.
Both the melting point and the melting range are affected by the pressure of the environment.
3. Melting Point Diagrams of An Compound
Let’s have a detailed discussion about the melting points of alloys using the following diagrams.
 [1]
[1]
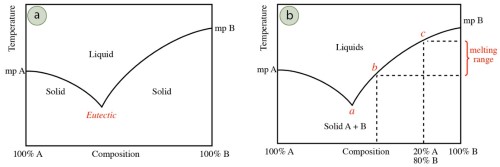
These 2 melting point diagrams manifest the melting range of the compound (a mixture of metal A and metal B).
- The far left side of the graph represents where only pure metal A exists, while the far right side represents where pure metal B exists.
- The line in the diagram is a clear manifestation of the solid-liquid transition. The melting point decreases as the blend becomes impure. The lowest melting point appears in the middle of the graph.
- The intersection point is called the eutectic point. Some systems do not have any eutectic points. Some have multiple ones.
- However, the actual situation is a little different. Before the temperature reaches the eutectic points, metal B has already begun to melt, and both A and B could be found at this point.
- Another conclusion that can be drawn is that the eutectic point of an alloy is generally lower than the melting point of the pure metal of its constituent elements.
4. The Melting Point of An Alloy Is Lower - Why?
Most alloys have lower melting points compared with their constituent metals because their atoms have more regular arrangements and stronger bonds. You can have a better understanding of this phenomenon after learning about the process of melting.
In the beginning, the atoms of crystalline materials like metals abide by a 3-dimensional arrangement, namely a crystalline lattice. They are held together closely by strong lattice forces. These particles absorb more energy and move more strongly. Finally, the tough crystalline structure is destroyed, and the solid material melts. The more energy is needed, the higher the melting point is. There are several melting points data for metals and alloys below:
Table 1 Melting Point of Some Metals and Alloys
| Element | Melting Point (°C) | Melting Point (°F) |
| Aluminum | 660.32 | 1220.58 |
| Copper | 1084.62 | 1984.32 |
| Iron | 1538 | 2800 |
| Nickel | 1453 | 2651 |
| Silver | 961.78 | 1763.2 |
| Tin | 231.93 | 449.47 |
| Titanium | 1668 | 3034 |
| Zirconium | 1855 | 3371 |
| Aluminum-Iron Alloy | 1153 | 2107 |
| Aluminum-Nickel Alloy | 1385 | 2525 |
| Brass, Red | 990 - 1025 | 1810 - 1880 |
| Brass, Yellow | 905 - 932 | 1660 - 1710 |
| Bronze, Aluminum | 1027 - 1038 | 1881 - 1900 |
| Copper-Nickel Alloy | 1060-1240 | 1940-2264 |
| Iron, Cast | 1204 | 2200 |
| Iron, Wrought | 1482 | 2700 |
5. Other Differences between Alloys & Metals
Alloys differ from their constituent metals in the following aspects:
- Alloys are stronger than their constituent metals.
Brittle pure metals are combined to form harder and more durable alloys. Bronze (88% copper and 12% tin) is tougher than copper. It is more easily melted and can be cast easier.
- Alloys have better corrosion resistance than their constituent metals.
Pure metals are more easily to be affected by corrosion. So alloys are created to fight against corrosion. The percentages of aluminum, iron, and nickel in aluminum bronze are from 6% to 12%. Aluminum bronze has high corrosion resistance, so it is a great option to make devices like pumps and valves exposed to corrosive fluids.
6. Conclusion
Alloys are a mixture of metals and other substances to acquire desired qualities such as toughness, durability, and corrosion resistance. A majority of alloys have lower melting points than their constituent metals. Hope that you can have a better comprehension of alloys and metals after reading this article. Advanced Refractory Metals provides high-quality metals and alloys of different shapes and sizes at competitive prices within a short time. Welcome to visit our site for more messages.
Reference:
[1]Nichols, L. (2020). Melting Point Diagrams [Photograph]. https://chem.libretexts.org/Courses/SUNY_Oneonta/Chem_221%3A_Organic_Chemistry_I_(Bennett)/2%3ALab_Textbook_(Nichols)/06%3A_Miscellaneous_Techniques/6.01%3A_Melting_Point/6.1C%3A__Melting_Point_Theory
{{item.content}}
LEVE A REPLY
{{item.children[0].content}}
{{item.content}}






