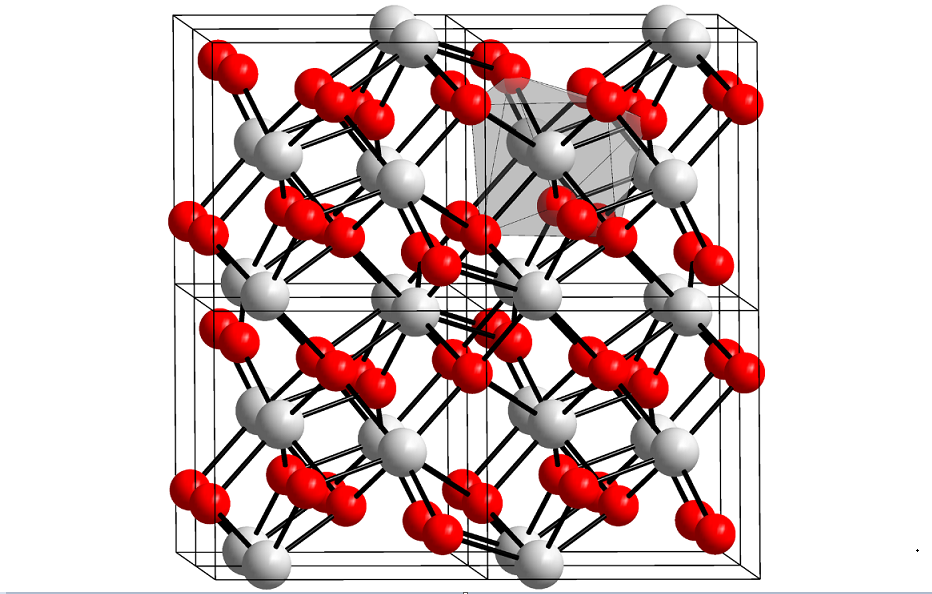
Hafnium Oxide and Its Structure And Applications Hafnium oxide is the inorganic compound of the formula HfO2. Also known as hafnia, this colorless solid is one of the most common and stable compounds of hafnium. It is an electrical isolator with a bandgap of 5.3 ~ 5.7 eV. Hafnium dioxide is an intermediate in some […]
Tags: Hafnium oxide, hafnium oxide application, hafnium oxide structure, Hafnium Oxide's Applications, Hafnium Oxide's Structure, HfO2, The Applications Of Hafnium Oxide

Applications of Titanium Alloy in the Automobile Industry Titanium has the advantages of low density, high specific strength, and good corrosion resistance. Titanium used in automobiles can greatly reduce the quality of the body, reduce fuel consumption, improve the working efficiency of the engine, improve the environment, and reduce noise. However, the expensive price of […]
Tags: Applications of Titanium Alloy, pure titanium, titanium, titanium alloy, Titanium Alloy in Automobile Industry, Titanium alloy parts, titanium rod

Why Is Zirconium A Transition Metal? When of sufficient purity, zirconium is soft and ductile. Zirconium a transition metal has good corrosion resistance and low absorption capacity for thermal neutrons. These properties are desirable in materials used for certain parts of nuclear reactors. The great increase in the production of zirconium since 1945 has been […]
Tags: Advanced Refractory Metals, alloys of zirconium, ARM, cast ingots of zirconium, Chromium, Hafnium, Hafnium oxide, powder-metallurgical process, Refractory Metals, Tungsten, Why Is Zirconium A Transition Metal?, Zircaloy, Zirconium, Zirconium A Transition Metal, zirconium metal

What Hafnium Is Used For? Hafnium occurs in zirconium ores. Because this metal must be removed from “reactor-grade” zirconium, the production of hafnium is largely dependent on zirconium production, as discussed later. Its melting point is 4032℉. However, it has not been available in sufficiently large quantities to have any extensive elevated-temperature applications other than […]
Tags: Advanced Refractory Metals, Hafnium, Hafnium oxide, hafnium product, Refractory Metals, What Hafnium Is Used For?, X-ray tubes

Why Platinum Is The Most Expensive Metal? The platinum-group metals can be described as refractory metals because of their high melting points. This group comprises platinum, osmium, iridium, ruthenium, rhodium, and palladium, i.e., metals that have properties similar to those of platinum. The metals in this group are quite scarce and expensive, such as platinum, […]
Tags: palladium, Platinum, platinum group, platinum metals, platinum wire

What Can Rhenium Be Used For? Rhenium is classified as a scarce refractory metal. It has the second-highest melting point of any of the metals and has a relatively high density. Rhenium is produced as a powder from molybdenite, which also contains rhenium sulfide. The rhenium powder is consolidated by pressing and resistance sintering in […]
Tags: Advanced Refractory Metals, Refractory Metals, rhenium, Rhenium bars, rhenium powder, thermocouple alloys, What Can Rhenium Be Used For?

How Does Chromium Work? In this article, we will take a look at how chromium works. The melting point of chromium is the lowest of the abundant refractory metals, but it is more than 700 degrees above that of iron. The density of chromium is slightly less than that of iron. At elevated temperatures, it […]
Tags: Abundant Refractory Metals, Advanced Refractory Metals, Chromium, Chromium metal, How Chromium Works?, Refractory Metals

Why Is Vanadium A Transition Metal? The melting point of vanadium is about 1000 degrees lower than that of columbium, so there is much less interest in vanadium for high-temperature applications than in abundant refractory metals. Pure vanadium has only recently become available in quantities large enough for thorough studies of its physical and mechanical properties […]
Tags: Abundant Refractory Metals, Pure vanadium, Refractory Metals, Titanium alloys, Transition Metal, Vanadium, Vanadium A Transition Metal, vanadium columbium, Why Is Vanadium A Transition Metal?

What Is Niobium Used For In Everyday Life? Niobium has a melting point of 4474℉, which is 256 degrees lower than that of molybdenum. The development of niobium metal and its alloys for elevated-temperature structural applications was started only a few years ago, but considerable progress has been made since then. One factor that has been […]
Tags: Niobium, Niobium alloys, niobium metal, Pure niobium, Refractory Metal, Refractory Metals, What Is Niobium, What Is Niobium Used For In Everyday Life?

Will Molybdenum Combine with Other Elements? Molybdenum. As shown in Table 2, the melting temperature of molybdenum is 695 degrees below that of tantalum. Because of its high melting point and relative abundance in the United States, it was the first of the refractory metals considered in this country for high-temperature structural applications. The primary […]
Tags: Advanced Refractory Metals, Molybdenum, Molybdenum Combine with Other Elements, molybdenum metal, molybdenum-tungsten alloys, Refractory Metals, tantalum, Will Molybdenum Combine with Other Elements?
Copyright © 1994-2024 Advanced Refractory Metals owned by Oceania International LLC, All Rights Reserved.